Muskeg
Overview
Muskeg is a nutrient-poor peatland characterized by acidic, saturated peat, and scattered or clumped, stunted conifer trees set in a matrix of sphagnum mosses and ericaceous shrubs. Black spruce (Picea mariana) and tamarack (Larix laricina) are typically the most prevalent tree species. The community primarily occurs in large depressions on glacial outwash and sandy glacial lakeplains. Fire occurs naturally during periods of drought and can alter the hydrology, mat surface, and floristic composition of muskegs. Windthrow, beaver flooding, and insect defoliation are also important disturbance factors that influence species composition and structure.
Rank
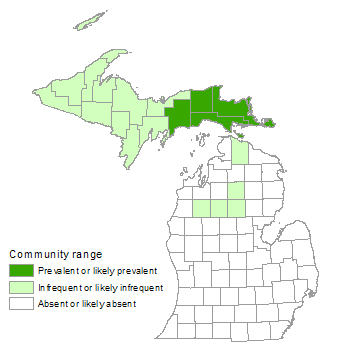
Global Rank: G4G5 - Rank is uncertain, ranging from apparently secure to secure
State Rank: S3 - Vulnerable

Landscape Context
Muskegs primarily occur on broad, flat areas or mild depressions of glacial outwash and glacial lakeplains but can also be found in large depressions on pitted outwash and moraines. Peatlands occurring on former glacial lakebeds and drainageways tend to be more extensive than kettle peatlands, which are limited in area by the size of the glacial ice-block that formed the basin. Muskegs within large wetland complexes typically occur adjacent to other peatland communities, often grading into bog, patterned fen, poor fen, poor conifer swamp, and/or rich conifer swamp. More minerotrophic systems such as northern fen, northern shrub thicket, northern wet meadow, rich conifer swamp, and hardwood-conifer swamp can occur along the outer margins of muskegs where groundwater seepage from the adjacent uplands is prevalent. Muskegs frequently occur adjacent to lakes and meandering streams (usually less than third order), which often weave along the margins of muskegs and through the adjoining minerotrophic wetlands. Upland community types that often occur adjacent to muskegs include dry northern forest, dry-mesic northern forest, and mesic northern forest. Sandy ridges dominated by white pine, red pine, and/or jack pine often occur within peatland complexes, especially in the eastern Upper Peninsula where these low ridges represent former transverse dunes.
Soils
The organic soils of muskegs are composed of peat, which forms a continuous mat ranging in thickness from one to eight meters but is typically one to three meters deep and overlays sand. The depth of peat and soil moisture increases with peatland age and can vary within a site. Peat depth is typically greatest near the center of a peatland and decreases toward the margin or in areas with groundwater influence. The rooting zone within muskegs is quite shallow, typically confined to the uppermost 15 cm of the surface peat, where there is sufficient oxygen to maintain aerobic respiration. The surface peats of muskegs are dominated by saturated fibric peat, which is loosely compacted and spongy, contains partially decomposed sphagnum moss with fragments of wood and occasionally sedge, and like the surface water, is extremely acidic, cool, and characterized by low nutrient availability and low oxygen levels. Peat composition changes with depth and varies with the successional history of a given peatland. Generally, fiber content and hydraulic conductivity decrease with depth. Deep humidified peats can effectively seal basins and create a perched water table.
Natural Processes
Muskegs are ombrotrophic to weakly minerotrophic peatlands, receiving inputs of water and nutrients primarily from ion-poor precipitation. Saturated and inundated conditions inhibit organic matter decomposition and allow for the accumulation of peat. Under cool, anaerobic, and acidic conditions, the rate of organic matter accumulation exceeds organic decay. Once sphagnum mosses become established on the peat mat, they maintain and enhance saturated, acidic, and cool conditions, which in turn promote continued peat development.
Development and expansion of peatlands occur via two distinct processes: lake-filling and paludification. Lake-filling occurs in small lakes with minimal wave action, where gradual peat accumulation results in the development of a peat mat that can fill the basin or occur as a floating mat or grounded mat. Paludification is the blanketing of terrestrial systems (often forests) by the overgrowth of sphagnum mosses and peatland vegetation. Paludified peatlands typically develop on flat areas, especially lakeplains, where peat builds vertically and spreads horizontally. The lateral expansion of peatland into forested systems can lead to the conversion to muskeg since thick sphagnum mats can limit tree establishment and growth. For both lake-filling and paludification, peat accumulates above the water table and the peatland becomes isolated from the influence of groundwater.
Once established, muskeg can persist for hundreds of years given stable hydraulic conditions and the lack of fire, which can burn the canopy and organic soils. Fire, which is an infrequent but important disturbance factor within peatlands, controls plant population dynamics by initiating and terminating succession. Estimates of fire return intervals for forested peatlands range widely from over a hundred to several hundred years in fire-prone landscapes to several hundred to over a thousand years for muskegs embedded within fire-protected landscapes. Fire severity and frequency in muskegs are closely related to climatic change and fluctuations in water level. Prolonged periods of drought and lowered water table can allow the surface peat to dry out sufficiently to burn, killing existing vegetation and occasionally exposing mineral soil. Low-severity surface fires in open peatlands can contribute to their maintenance by killing encroaching trees, promoting sprouting of ericaceous shrubs, and minimally impacting sphagnum moss cover.
Changes in the water and nutrient regimes of peatlands result in significant modification of species composition and abundance. Beaver, through their dam-building activities, can cause substantial hydrologic change to peatland systems, causing either flooding or the lowering of the water table depending on the location of the peatland in relation to the dam. Behind a beaver dam the water table is higher, while below it, drier conditions are generated. Short periods of flooding can cause needle chlorosis, necrotic needle tips, and decreased shoot and root growth of swamp conifers due to low oxygen concentration and nutrient availability in the rooting medium from water logging. Prolonged flooding of poor conifer swamps can result in the death of the canopy trees and the conversion to muskegs and bogs or even open systems dominated by marsh and fen vegetation. The lowering of the water table through beaver damming or climatic changes can result in the conversion of open peatlands to northern shrub thicket or poor conifer swamp.
Tree survival in muskegs is limited by windthrow, insects, and parasites. Trees growing in muskeg are particularly susceptible to windthrow because saturated sphagnum peat provides a poor substrate for anchoring trees. Small-scale wind disturbance, along with insect herbivory, contributes to the structural diversity of muskegs by generating moderate pit and mound microtopography, standing snags, and coarse woody debris that is quickly enveloped by sphagnum mosses. The plant parasite dwarf mistletoe (Arceuthobium pusillum) can increase the mortality of black spruce. Three insect defoliators are prevalent in peatlands: larch sawfly (Pristophora erichsonii), larch casebearer (Coleophora laricella), and spruce budworm (Choristoneura fumiferana). Spruce budworm defoliates both black spruce and balsam fir (Abies balsamea) but tends to be more detrimental to the latter. Tamarack growing in peatlands often suffers from repeated defoliation by larch sawfly. Although a more recent arrival in Michigan, the larch casebearer is beginning to cause heavy defoliation to tamarack, especially in the eastern and central Upper Peninsula.
Vegetation
Muskegs are characterized by a poor herbaceous layer dominated by sedges and a hummocky carpet of sphagnum moss, low ericaceous, evergreen shrubs, and widely scattered or clumped, stunted conifers. Floristically, muskegs are homogenous and of limited diversity, exhibiting remarkably uniform structure and composition across their wide range. The continuous moss layer of muskegs is typically dominated by sphagnum mosses, especially Sphagnum angustifolium, S. centrale, S. fuscum, S. magellanicum, and S. recurvum. Additional mosses can include S. capillaceum, S. capillifolium, S. compactum, S. cuspidatum, S. papillosum, S. recurvum, S. rusowii, and Drepanocladus aduncus. The hummock and hollow microtopography of muskeg allows for high levels of bryophyte diversity since individual species of sphagnum occur at specific elevations, exhibiting resource partitioning. The herbaceous layer of muskegs is depauperate and dominated by cyperaceous plants. Several sedges that are characteristic of muskegs include coastal sedge (Carex exilis), mud sedge (C. limosa), wiregrass sedge (C. lasiocarpa), few-flower sedge (C. pauciflora), few-seed sedge (C. oligosperma), and three-seeded sedge (C. trisperma). Additional graminoids found in muskegs include twig-rush (Cladium mariscoides), three-way sedge (Dulichium arundinaceum), narrow-leaved cotton-grass (Eriophorum angustifolium), sheathed cotton-grass (E. vaginatum), tawny cotton-grass (E. virginicum), rattlesnake grass (Glyceria canadensis), and white beak-rush (Rhynchospora alba). The following are prevalent muskeg herbs: bog aster (Oclemena nemoralis), goldthread (Coptis trifolia), fireweed (Chamerion angustifolium), fringed willow-herb (Epilobium ciliatum), wild blue flag (Iris versicolor), bog buckbean (Menyanthes trifoliata), starry false Solomon’s seal (Maianthemum stellatum), false mayflower (M. trifolium), starflower (Trientalis borealis), and common bog arrow-grass (Triglochin maritima). Insectivorous plants, including round-leaved sundew (Drosera rotundifolia), spoon-leaf sundew (Drosera intermedia), pitcher-plant (Sarracenia purpurea), and flat-leaved bladderwort (Utricularia intermedia), are common features of muskegs. The shrub layer of muskegs is dominated by low, ericaceous shrubs, with leatherleaf (Chamaedaphne calyculata) being the most prevalent species. In addition to leatherleaf, the following primarily heath shrubs are important components of muskegs: bog rosemary (Andromeda glaucophylla), creeping snowberry (Gaultheria hispidula), wintergreen (G. procumbens), sheep-laurel (Kalmia angustifolia), bog laurel (K. polifolia), Labrador tea (Rhododendron groenlandicum), bog willow (Salix pedicellaris), low sweet blueberry (Vaccinium angustifolium), Canada blueberry (V. myrtilloides), large cranberry (V. macrocarpon), and small cranberry (V. oxycoccos). The tall shrub layer of muskegs is less dense than the low shrub layer and is often restricted to the periphery of the community. Tall shrubs typical of muskegs include black chokeberry (Aronia prunifolia), mountain holly (Ilex mucronata), pussy willow (Salix discolor), meadowsweet (Spiraea alba), steeplebush (S. tomentosa), and wild-raisin (Viburnum cassinoides). Trees within muskegs are stunted, usually no taller than two to three meters, and widely scattered or clumped. Tree cover is typically between 10 and 25%. The most common are black spruce and tamarack, with jack pine (Pinus banksiana), white pine (P. strobus), and red pine (P. resinosa) as occasional associates.
For information about plant species, visit the Michigan Flora website.
Plant Lists
Graminoids
- sedges (Carex oligosperma, C. pauciflora, C. trisperma, and others)
- cotton-grasses (Eriophorum angustifolium, E. vaginatum, and E. virginicum)
- white beak-rush (Rhynchospora alba)
Forbs
- goldthread (Coptis trifolia)
- pink lady-slipper (Cypripedium acaule)
- spatulate-leaved sundew (Drosera intermedia)
- round-leaved sundew (Drosera rotundifolia)
- false mayflower (Maianthemum trifolium)
- bog buckbean (Menyanthes trifoliata)
- bog aster (Oclemena nemoralis)
- pitcher-plant (Sarracenia purpurea)
- arrow-grass (Scheuchzeria palustris)
- starflower (Trientalis borealis)
Mosses
- ribbed bog moss (Aulacomnium palustre)
- big red stem moss (Pleurozium schreberi)
- pohlia moss (Pohlia nutans)
- sphagnum mosses (Sphagnum spp.)
Shrubs
- bog rosemary (Andromeda glaucophylla)
- black chokeberry (Aronia prunifolia)
- leatherleaf (Chamaedaphne calyculata)
- creeping snowberry (Gaultheria hispidula)
- wintergreen (Gaultheria procumbens)
- mountain holly (Ilex mucronata)
- bog laurel (Kalmia polifolia)
- Labrador-tea (Rhododendron groenlandicum)
- low sweet blueberry (Vaccinium angustifolium)
- large cranberry (Vaccinium macrocarpon)
- Canada blueberry (Vaccinium myrtilloides)
- small cranberry (Vaccinium oxycoccos)
Trees
- tamarack (Larix laricina)
- black spruce (Picea mariana)
- jack pine (Pinus banksiana)
- red pine (Pinus resinosa)
- white pine (Pinus strobus)
Noteworthy Animals
In general, the population of animals is low in muskegs because of the low productivity of peatland plants, unpalatability of the vegetation, and high acidity of the peat. Bogs and muskegs provide important habitat for small mammals such as short-tailed shrew (Blarina brevicauda), beaver (Castor canadensis), meadow vole (Microtus pennsylvanicus), mink (Mustela vison), muskrat (Ondatra zibethicus), and masked shrew (Sorex cinereus). Both muskrats and beaver can profoundly influence the hydrology of open peatlands. Muskrats create open water channels through the peat and beavers can cause substantial flooding through their dam-building activities.
Rare Plants
- Carex heleonastes (Hudson Bay sedge, state endangered)
- Carex wiegandii (Wiegand's sedge, state threatened)
- Empetrum nigrum (black crowberry, state threatened)
- Rubus acaulis (dwarf raspberry, state endangered)
Rare Animals
- Alces alces (moose, state threatened)
- Ardea herodias (great blue heron, protected by the Migratory Bird Treaty Act of 1918)
- Boloria freija (Freija fritillary, state special concern)
- Boloria frigga (Frigga fritillary, state special concern)
- Botaurus lentiginosus (American bittern, state special concern)
- Canis lupus (gray wolf, state threatened)
- Circus cyaneus (northern harrier, state special concern)
- Clemmys guttata (spotted turtle, state threatened)
- Coturnicops noveboracensis (yellow rail, state threatened)
- Cryptotis parva (least shrew, state threatened)
- Emydoidea blandingii (Blanding’s turtle, state special concern)
- Erebia discoidalis (red-disked alpine, state special concern)
- Falcipennis canadensis (spruce grouse, state special concern)
- Falco columbarius (merlin, state threatened)
- Haliaeetus leucocephalus (bald eagle, state threatened)
- Lynx canadensis (lynx, state endangered)
- Pandion haliaetus (osprey, state threatened)
- Picoides arcticus (black-backed woodpecker, state special concern)
- Somatochlora incurvata (incurvate emerald, state special concern)
- Sorex fumeus (smoky shrew, state special concern)
- Tympanuchus phasianellus (sharp-tailed grouse, state special concern)
- Williamsonia fletcheri (ebony boghaunter, state special concern)
Biodiversity Management Considerations
The primary mechanism for preserving muskegs is to maintain their hydrology. A serious threat to muskeg hydrology is posed by off-road vehicle traffic, which can significantly alter hydrology through rutting. Reducing access to peatland systems will help decrease detrimental impacts. Avoiding the construction of new roads that traverse peatlands will help prevent unintended hydrologic alteration. The installation and maintenance of culverts under roads passing through peatlands can avert flooding and drying. Resource managers operating in uplands and forested peatlands adjacent to muskegs should take care to minimize the impacts of management to hydrologic regimes, especially increased surface flow. This can be accomplished by establishing a no-cut buffer around muskegs and avoiding road construction and complete canopy removal in stands immediately adjacent to muskegs.
Anthropogenic disturbance has decreased the extent of peatlands and dramatically altered many occurrences. Turn-of-the-century logging of tamarack, black spruce, and cedar from peatland systems favored the conversion of forested peatlands to open, ombrotrophic bogs and muskegs. Historically, widespread slash fires followed logging, converting poor conifer swamp to open bogs or muskegs or destroying the peat and converting peatlands to mineral soil wetlands. Beginning in the 1920s, effective fire control reduced the acreage of fires ignited by humans or lightning. In landscapes where frequent fire was the prevalent disturbance factor, fire suppression has led to the conversion of open bogs and muskegs to closed-canopy peatlands and the maintenance of closed-canopy poor conifer swamps. Peat mining and cranberry farming have degraded numerous peatlands throughout the region. In addition to direct impacts to vegetation, alteration of peatland hydrology from road building, creation of drainage ditches and dams, and runoff from logging and agriculture has led to significant changes in peatland composition and structure.
Peatland vegetation is extremely sensitive to minor changes in water levels and chemistry. Succession to more minerotrophic wetlands can occur as the result of increased alkalinity and raised water levels, which can cause the increased decomposition of acidic peats. Flooding of muskegs and poor conifer swamps can cause the death of canopy trees and the conversion of forested peatland to open wetlands. Flooding of poor conifer swamps can result in the conversion to muskeg. Roads and highways traversing through large peatland complexes, especially in the Upper Peninsula, have caused the blockage of drainage (impoundment of water) and the alteration of muskegs and poor conifer swamps to open peatlands. Conversely, lowering of water tables from drainage can allow for tree and shrub encroachment into open bogs and muskegs and the eventual succession to closed-canopy peatland. The dependence of muskegs on precipitation for nutrients and water makes them especially susceptible to acid rain and air pollution. Atmospheric deposition can contribute nitrogen, sulphur, calcium, and heavy metals to peatlands. Eutrophication from pollution and altered hydrology can detrimentally impact peatlands by generating conditions favorable for invasive plant species. Particularly aggressive invasive species that may threaten the diversity and community structure of muskeg include glossy buckthorn (Frangula alnus), narrow-leaved cat-tail (Typha angustifolia), hybrid cat-tail (Typha xglauca), reed canary grass (Phalaris arundinacea), and reed (Phragmites australis subsp. australis). At present, most of these invasive species appear to be restricted to the margins of muskegs, where they occur in moats or ditches along roads and trails that border the community. Monitoring and control efforts to detect and remove invasive species before they become widespread are critical to the long-term viability of muskeg.
Variation
Muskegs occurring on glacial lakeplains and outwash plains tend to be more extensive than those occurring in kettle depressions, which are limited in area by the size of the glacial ice-block that formed the basin.
Similar Natural Communities
Bog, northern fen, patterned fen, poor fen, poor conifer swamp, rich tamarack swamp, and rich conifer swamp.
Places to Visit
- Betchler Tamarack Flats Candidate Research Natural Area, Hiawatha National Forest, Chippewa Co.
- Dawson Creek Muskeg, Newberry State Forest Management Unit and The Nature Conservancy (Two-Hearted River Forest Preserve), Luce Co.
- Nine Mile Muskeg, Roscommon State Forest Management Unit, Roscommon Co.
- No Muskeg for Old Men, Newberry State Forest Management Unit, Luce Co.
- Prison Camp Muskeg, Tahquamenon Falls State Park and Newberry State Forest Management Unit, Luce Co. and Chippewa Co.
- Sylvania, Sylvania Wilderness and Recreation Area, Ottawa National Forest, Gogebic Co.
Relevant Literature
- Boelter, D.H., and E.S. Verry. 1977. Peatland and water in the northern Lake States. North Central Forest Experiment Station. USDA, Forest Service General Technical Report NC-31. 26 pp.
- Bridgham, S.D., J. Pastor, J.A. Janssens, C. Chapin, and T.J. Malterer. 1996. Multiple limiting gradients in peatlands: A call for a new paradigm. Wetlands 16(1): 45-65.
- Cohen, J.G. 2006. Natural community abstract for muskeg. Michigan Natural Features Inventory, Lansing, MI. 20 pp.
- Curtis, J.T. 1959. The vegetation of Wisconsin. University of Wisconsin Press, Madison, WI. 657 pp.
- Dansereau, P., and F. Segadas-Vianna. 1952. Ecological study of the peat bogs of eastern North America. I. Structure and evolution of vegetation. Canadian Journal of Botany 30: 490-520.
- Eggers, S.D., and D.M. Reed. 1997. Wetland plants and plant communities of Minnesota and Wisconsin. U.S. Army Corps of Engineers, St. Paul, MN. 263 pp.
- Futyma, R.P., and N.G. Miller. 1986. Stratigraphy and genesis of the Lake Sixteen peatland, northern Michigan. Canadian Journal of Botany 64: 3008-3019.
- Gates, F.C. 1942. The bogs of northern Lower Michigan. Ecological Monographs 12(3): 213-254.
- Gignac, L.D., L.A. Halsey, and D.H. Vitt. 2000. A bioclimatic model for the distribution of sphagnum-dominated peatlands in North America under present climatic conditions. Journal of Biogeography 27(5): 1139-1151.
- Glaser, P.H., J.A. Janssens, and D.I. Siegel. 1990. The response of vegetation to chemical and hydrological gradients in the Lost River Peatland, northern Minnesota. Journal of Ecology 78(4): 1021-1048.
- Halsey, L.A., and D.H. Vitt. 2000. Sphagnum-dominated peatlands in North America since the last glacial maximum: Their occurrence and extent. The Bryologist 103(2): 334-352.
- Heinselman, M.L. 1963. Forest sites, bog processes, and peatland types in the Glacial Lake Region, Minnesota. Ecological Monographs 33(4): 327-374.
- Heinselman, M.L. 1970. Landscape evolution, peatland types, and the environment in the Lake Agassiz Peatland Natural Area, Minnesota. Ecological Monographs 40(2): 235-261.
- Janssen, C.R. 1967. A floristic study of forests and bog vegetation, northwestern Minnesota. Ecology 48(5): 751-765.
- Karlin, E.F., and L.C. Bliss. 1984. Variation in substrate chemistry along microtopographical and water-chemistry gradients in peatlands. Canadian Journal of Botany 62: 142-153.
- Miller, N. 1981. Bogs, bales, and BTU’s: A primer on peat. Horticulture 59: 38-45.
- Miller, N.G., and R.P. Futyma. 1987. Paleohydrological implications of Holocene peatland development in northern Michigan. Quaternary Research 27: 297-311.
- Schwintzer, C.R. 1981. Vegetation and nutrient status of northern Michigan bogs and conifer swamps with a comparison to fens. Canadian Journal of Botany 59: 842-853.
- Schwintzer, C.R, and T.J. Tomberlin. 1982. Chemical and physical characteristics of shallow ground waters in northern Michigan bogs, swamps, and fens. American Journal of Botany 69(8): 1231-1239.
- Taylor, S.J., T.J. Carleton, and P. Adams. 1988. Understory vegetation change in a Picea mariana chronosequence. Vegetatio 73(2): 63-72.
- Vitt, D.H., H. Crum, and J.A. Snider. 1975. The vertical zonation of Sphagnum species in hummock-hollow complexes in northern Michigan. Michigan Botanist 14(4): 190-200.
- Vitt, D.H., and N.G. Slack. 1984. Niche diversification of Sphagnum relative to environmental factors in northern Minnesota peatlands. Canadian Journal of Botany 62(7): 1409-1430.
- Vogl, R.J. 1964. The effects of fire on a muskeg in northern Wisconsin. Journal of Wildlife Management 28(2): 317-329.
- Zoltai, S.C., and D.H. Vitt. 1995. Canadian wetlands: Environmental gradients and classification. Vegetatio 118: 131-137.
For a full list of references used to create this description, please refer to the natural community abstract for Muskeg.
More Information
Citation
Cohen, J.G., M.A. Kost, B.S. Slaughter, D.A. Albert, J.M. Lincoln, A.P. Kortenhoven, C.M. Wilton, H.D. Enander, and K.M. Korroch. 2020. Michigan Natural Community Classification [web application]. Michigan Natural Features Inventory, Michigan State University Extension, Lansing, Michigan. Available https://mnfi.anr.msu.edu/communities/classification. (Accessed: March 4, 2026).
Kost, M.A., D.A. Albert, J.G. Cohen, B.S. Slaughter, R.K. Schillo, C.R. Weber, and K.A. Chapman. 2007. Natural Communities of Michigan: Classification and Description. Michigan Natural Features Inventory, Report No. 2007-21, Lansing, MI.